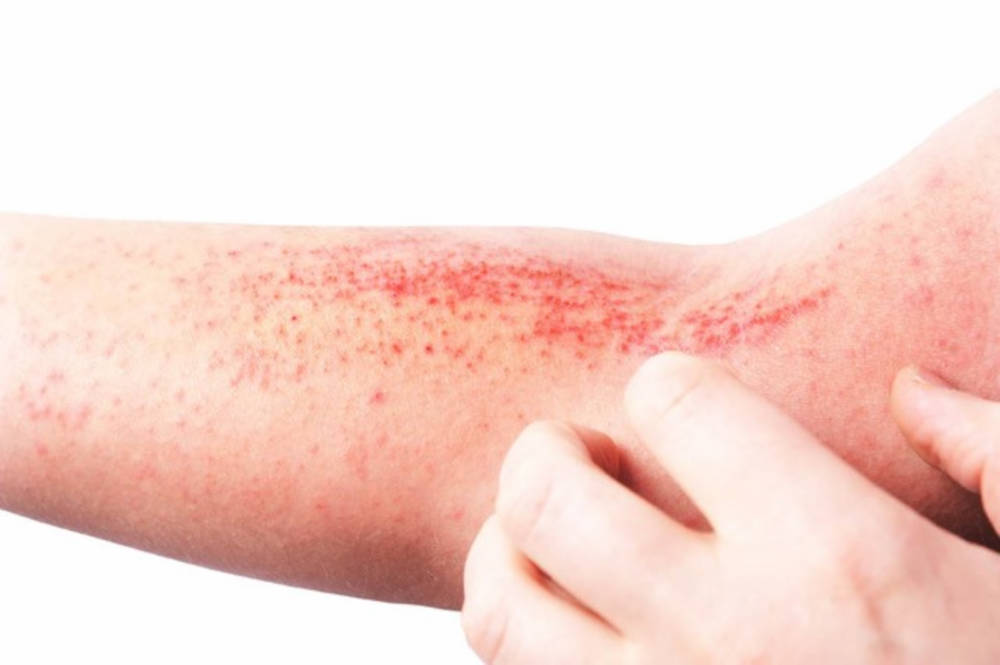
The U.S. Food and Drug Administration (FDA) has approved Eli Lilly and Company’s EBGLYSS (lebrikizumab-lbkz), a targeted IL-13 inhibitor, for the treatment of adults and children 12 years of age and older who weigh at least 88 pounds (40 kg) with moderate-to-severe atopic dermatitis (eczema) that is not well controlled despite treatment with topical prescription therapies. Eczema inflammation under the skin can lead to symptoms seen and felt on the outside. EBGLYSS works by targeting eczema inflammation throughout the body that can lead to dry, itchy and irritated skin.
EBGLYSS 250 mg/2 mL injection can be used with or without topical corticosteroids and is dosed as a single monthly maintenance injection following the initial phase of treatment. The recommended initial starting dose of EBGLYSS is 500 mg (two 250 mg injections) at Week 0 and Week 2, followed by 250 mg every two weeks until Week 16 or later when adequate clinical response is achieved; after this, maintenance dosing is a single monthly injection (250 mg every four weeks).
“Patients still struggle to control their moderate-to-severe atopic dermatitis with currently available therapies. Many experience poor long-term disease control, and severe itch can significantly impact their daily lives,” said Jonathan Silverberg, M.D., Ph.D., M.P.H., professor of dermatology at George Washington University School of Medicine and Health Sciences in Washington, DC, and first author of the New England Journal of Medicine manuscript summarizing EBGLYSS clinical trials. “Today’s FDA approval of EBGLYSS is a big win for patients, as we now have a new first-line biologic treatment option for moderate-to-severe disease when topical prescriptions aren’t enough.”
The approval was based on results from the ADvocate 1, ADvocate 2, and ADhere studies, which included over 1,000 adults and children (aged 12 and older) with moderate-to-severe eczema who were unable to control their symptoms with topical prescription medicines. The primary endpoint for these studies was evaluated at 16 weeks and measured clear or almost clear skin (IGA 0,1).
In an average of two studies (ADvocate 1 and 2), 38 percent of people who took EBGLYSS achieved clear or almost-clear skin at 16 weeks (versus 12 percent with placebo) and 10 percent saw these results as early as four weeks. Of the people who experienced clear or almost-clear skin at Week 16, 77 percent maintained those results at one year with once-monthly dosing. Forty-eight percent of responders who were switched from EBGLYSS to placebo at Week 16 maintained these results at one year.
Similarly, in both studies, many people experienced itch relief with EBGLYSS. On average, 43 percent of people who took EBGLYSS felt itch relief at 16 weeks (compared to 12 percent who took placebo) and five percent felt relief as early as two weeks. Of the people who felt itch relief at Week 16, 85 percent still felt that relief at one year of treatment with monthly maintenance dosing. Sixty-six percent of responders who were switched from EBGLYSS to placebo at Week 16 maintained these results at one year.
“Eczema can affect people of all skin tones, ethnicities, genders and ages. Nearly 16.5 million adults in the U.S. have eczema, with 6.6 million experiencing moderate-to-severe symptoms like itchiness, dry and scaly skin, discoloration and rashes, which can lead to more scratching that may cause skin to crack and bleed,” said Kristin Belleson, President and CEO of the National Eczema Association. “The approval of EBGLYSS provides hope and promise for the eczema community and those still seeking lasting relief from disruptive symptoms.”
The most common side effects of EBGLYSS include eye and eyelid inflammation, such as redness, swelling and itching; injection site reactions and shingles (herpes zoster).
“People living with eczema have symptoms that can appear at the most inopportune times, creating unpredictability and impacting their everyday lives,” said Daniel Skovronsky, M.D., Ph.D., chief scientific officer and president of Lilly Research Laboratories, and president, Lilly Immunology. “Today’s approval allows people the opportunity to reimagine life with eczema as EBGLYSS offers a targeted approach to reduce a main cause of eczema inflammation. EBGLYSS provides long-lasting symptom relief with a convenient once-monthly maintenance dose.”
EBGLYSS was approved for use by the European Commission in 2023, as well as in Japan in January 2024 with additional markets expected later this year.
Lilly has exclusive rights for development and commercialization of EBGLYSS in the U.S. and the rest of the world outside Europe. Lilly’s partner Almirall S.A. has licensed the rights to develop and commercialize EBGLYSS for the treatment of dermatology indications, including eczema, in Europe.